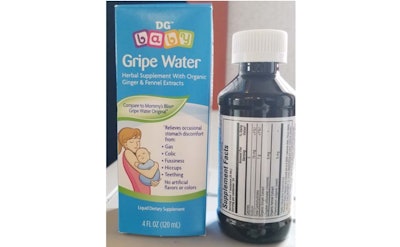
Kingston Pharma, LLC is voluntarily recalling all lots of the DG Baby Gripe Water herbal supplement with organic ginger and fennel extracts due to the presence of an undissolved, citrus flavonoid ingredient. Use of the product is not considered hazardous, but could result in difficulty when swallowing. To date, Kingston Pharma LLC has received one report of a one-week old infant having difficulty when swallowing the product and three complaints attributed to the undissolved citrus flavonoid.
The product is administered orally to infants and adults. It is packaged in four-ounce amber bottles, white plastic caps with safety seals and provided with an oral syringe. The product was distributed throughout the United States by Dollar General. The recall is being conducted in conjunction with the U.S. Food and Drug Administration.





















